| |
This activity asks students to evaluate
the usefulness of competing / complementary
models in two different contexts by examining
whether the models can explain data.
|
|
| |
| This activity comprises
of two related tasks. In both cases, the task concerns
comparing two ‘competing’ models: |
| |
| Task |
'Competing' model of |
Judged
against |
| Choosing
between models 1 |
Particle
theory
(Model 1 vs Model 2)
|
Application to explaining
common phenomena |
| Choosing
between models 2 |
Ionic bonding
(Model A vs Model B)
|
Application
to explaining properties of NaCl |
|
|
| |
| In each case the students,
working in groups, consider the merits of alternative
models in providing explanations. The second task
also includes some simple practical work, making
observations. The groups are provided with sets
of cards with features of the models, and the phenomena/properties
to be explained, to sort during the tasks. |
| |
| Rationale for the
activity |
| |
| This activity reinforces
the central role of modelling in science, and the
way that models are used in science to support explanations.
In the two tasks students are asked to consider
the merits of alternative models in explaining data.
The tasks have been designed to generate discussion,
and it is not intended that students will be able
to simply select a ‘better’ model that fits all
the data. This is especially the case in Task 1,
‘Using the particle model’, where they are briefed
that for each phenomenon they may decide that: |
| |
• one of the models
is more useful in explaining the phenomenon
• both of the models are useful in explaining the
phenomenon
• neither of the models are useful in explaining
the phenomenon |
| |
| Using the particle model |
| |
Task 1 asks students
to compare the very simple ‘hard billiard ball’
type model of particles often introduced early in
secondary education (Model 1), with a more complex
model of molecules are having internal structure,
fuzzy edges, and the possibility of interacting
with other molecules (Model 2).
Students are asked to consider a range of phenomena:
an ice cube melting; starch being converted to glucose
when mixed with saliva; steam produced from boiling
water; salt dissolving in water; a copper bar being
drawn out into a wire; a metal rod getting slightly
longer when it is heated; methane and oxygen |
| |
| Model one:
everything
is made up tiny particles, called molecules.
These particles can be considered to be hard
spheres, like tiny billiard balls, so that
when they collide they bounce off each other.
Unlike real billiard balls, they are ‘perfectly
elastic’: this means that no kinetic energy
is lost in the collision. In a solid the particles
are packed together so that no more will fit
– like a great many billiard balls arranged
in a regular pattern. The particles move about,
but they do not change. |
|
| |
| Model
two:
everything is made up of tiny particles,
called molecules. These molecules are themselves
comprised of smaller particles: one or more
positively charged core surrounded by a ‘cloud’
of negatively charged electrons. The electron
cloud makes the atoms ‘soft’ so that they
can overlap and ‘inter-penetrate’ one another.
The positive and negative charges in one molecule
will attract and repel the charged particles
in another molecule. The particles inside
molecules may be rearranged and exchanged
when molecules interact. |
|
| |
reacting in a Bunsen
flame; putty adhering to a wall; the pressure of
a gas increasing when the gas is heated; sugar dissolving
faster in hot tea than cold tea; magnesium burning
in the air to form magnesium oxide; current passing
through a copper wire; iron on a bicycle rusting
in a wet garden; chalk reacting quicker with acid
solution when the lumps are turned into powder;
a sample of radioactive material emitting alpha
radiation; the pressure of a gas increasing when
it is compressed; a spring returning to its original
length when a load is removed; ozone in the atmosphere
absorbing ultraviolet radiation; carbon dioxide
and water reacting in photosynthesis; very hot metal
glowing. (As with most of the ASCEND materials,
individual teachers are free to modify the list).
Clearly, neither model is suitable for explaining
all those phenomena that scientists use
particles ideas to explain - and in school science
different versions of particle theory are used at
different stages, and in different topics. Part
of the rationale of this activity is to help students
appreciate that models are used in this way, and
that often nature is too sophisticated to be represented
by a single simple model. A scientifically useful
model would need to incorporate features that enable
it to explain all the relevant data without being
internally inconsistent. |
| |
B1:
‘Model 1 really doesn’t have any . . . changes in
molecules . . . it’s purely physical . . . so any
of these involving chemical change are going to
be Model 2 . . . but the ones that involve physical
changes like an ice cube melting seem to . . . (be)
Model 1’ . . .
G: ‘but, can something be both . . . then?’
B2: ‘when salt dissolves in nature, it doesn’t interact
with the water, does it?’
B1: ‘not really . . . it would be a case of Model
1 . . . I think that’s basically it , if it’s chemical
change Model 2, if it’s physical change Model 1
. . . there’s probably some in there that involve
both . . .’
(Dialogue from
the ASCEND session: the delegates relate the task
to prior learning about chemical and physical change) |
| |
| Explaining the properties
of an ionic substance. |
| |
| Task 2 is slightly different
in that the two models being compared are not of
similar status in terms of school science. These
are models of ionic bonding. |
| |
| Model
A:
Table salt
is sodium chloride (NaCl) – a compound with
ionic bonding. Sodium has an atom with one
outer electron, and chlorine has an atom with
seven outer electrons. The atoms need full
outer shells. In ionic bonding the sodium
atom donates an electron to the chlorine atom,
so that both atoms can have full outer shells
(octet) of electrons. The
ionic bond is the transfer of electrons
that leads to a sodium chloride molecule.
Solid sodium chloride contains a very large
number of NaCl molecules. The sodium and chlorine
in a molecule are strongly held together by
ionic bonding, and the sodium chloride molecules
are also held together, by weak forces between
molecules. Each ion can only be bonded to
one other. This ‘valency’ of one is because
sodium only needs to lose one electron, and
chlorine only needs to gain one electron. |
|
| |
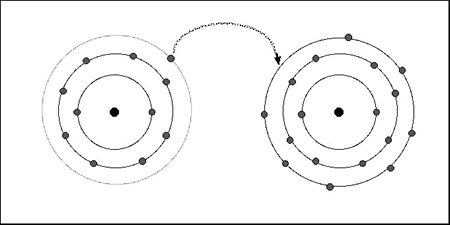 Figure 9.1:
Diagram representing ionic bonding in Model A
Figure 9.1:
Diagram representing ionic bonding in Model A
|
| |
| Model
B:
Table salt
is sodium chloride (NaCl) – a compound with
ionic bonding. Sodium chloride contains sodium
ions and chloride ions. Sodium ions have a
positive charge, and chloride ions have a
negative charge. In solid sodium chloride,
each sodium ion is surrounded by six chloride
ions, and each chloride ion is surrounded
by six sodium ions. The ions are attracted
together by electrical forces. The
ionic bond is the electrical attraction
that holds the ions together in the lattice.
Each ion is attracted strongly to the six
oppositely charged ions that surround it –
one above, one below, four in the same layer.
This ‘coordination number’ of six occurs because
of the way the two types of ion fit together
into a tightly bound lattice structure. |
|
| |
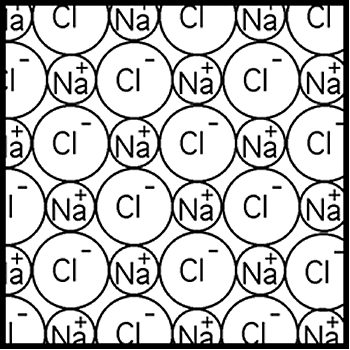 Figure 9.2:
Diagram of ionic bonding according to Model B
Figure 9.2:
Diagram of ionic bonding according to Model B
|
| |
Model B reflects
a level of scientific understanding that is suitable
for explaining properties of NaCl at a level appropriate
for students of this age group. Model A does not
reflect a scientific understanding, but is based
on the type of thinking about ionic bonding that
commonly develops in students by age 16 and which
has limited value in explaining the properties of
NaCl. Despite its clear inferiority, this type of
model has been found to be readily adopted by students,
perhaps even being intuitively attractive, and is
encouraged by vague (or even inaccurate) descriptions
of ionic bonding in many school level text books.
Therefore, although the evidence should
offer a clear preference for model B as having more
value, it is likely that many students will initially
find model A attractive, and the task is unlikely
to be considered trivial by most groups of students.
(Indeed the task would probably provide a very useful
review of prior learning in an A level or similar
course.) It is hoped this activity will either reinforce
the model being taught in school science, or prepare
the way for formal teaching later. Students who
completed the ASCEND activity on Explaining Science
(Activity 4) should also be alerted by the statement
that “the atoms need full outer shells” with its
anthropomorphic flavour.
The properties students are asked to explain are:
Sodium chloride is a hard substance; sodium chloride
cannot easily be compressed or stretched; solid
sodium chloride does not conduct electricity; sodium
chloride has a high melting temperature (1081K);
molten sodium chloride conducts electricity; sodium
chloride is colourless; sodium chloride dissolves
in water; sodium chloride solution conducts electricity;
sodium chloride forms crystals that are cubic; sodium
chloride tastes salty; Sodium chloride is brittle
(e.g. snaps rather than bends); a precipitate of
silver chloride is produced when sodium chloride
solution is mixed with a solution of silver nitrate;
sodium chloride crystals decrepitate when heated
strongly.
The second task includes two simple practical activities
that can also provide data to be explained using
the two models. Observing decrepitation is included
because it is a very simple phenomena, requiring
close observation, that fascinates many students:
careful strong heating of a sample of NaCl leads
to some of the grains suddenly appearing to ‘jump’
around. The effect can also be heard. If salt consisted
of pure, perfect, crystals of NaCl, then this behaviour
would not be expected: so decrepitation provides
a challenge to both models, and encourages students
to identify a feasible explanation. The effect is
due to the heating of small air pockets trapped
on crystallisation which lead to grain splitting
violently as the internal pressure increases: “Decrepitation
occurs when the internal pressure within the fluid
inclusion exceeds the strength of the host mineral”
(http://www.cas.gsu.edu/acres/sum2000/Fluid/page13.html,
accessed August 2006). |
| |
‘bear
in mind this is a theory . . . before, they’re sort
of quite cubical crystals because they’re in lattice
formation . . . so when you heat it, my theory is
that you get to a point at which the bonds between
the ion . . . the charged bonds in the lattice don’t
hold as much any more and the ions separate off
as elect . . . as molecules so then you get . .
. I’m not sure how good that is but . . . they go
more rounded because they’re no longer behaving
as a lattice in general because now the strongest
forces are between the . . . the molecules . . .
the ions in the molecule rather than the same or
throughout the lattice, so therefore they can form
whatever shape they like . . . so they go round.
. .’
(An ASCEND delegate conjectures
what is going on at the particle level during decreptiation) |
| |
| The second observation
task is an example of a precipitation (‘double decomposition’)
reaction. These reactions were commonly used in
school science at one time (as tests for halides,
sulphates etc.), but are not familiar to many students
today. Explaining the precipitation requires thinking
about the reaction at the level of particles, and
appreciating the ionic nature of the reactants.
The author knows from his own teaching that explaining
precipitation is found to be quite challenging by
some able students even after formal teaching about
ionic bonding. This observation provides data that
is therefore a test of the ability to apply model
B. |
| |
| Resources |
| |
| The following resources
are included on the CD: |
| |
| Resource |
Description |
Filename |
| Model descriptions |
Two pages. The
first offers two models of particles
(models 1, 2). The second offers
two models of ionic bonding (models
A, B), including a diagram for
each. |
Act
9 descriptions |
| Instructions |
4 pages: instructions
for
Choosing between models 1: Using
the particle model;
Choosing between models 2: Explaining
the properties of an ionic substance;
Decrepitation;
Precipitation.
|
Act
9 tasks |
| Particle model
1 cards |
Key points of model
1. To print out as cards* on e.g.
blue paper. |
Act
9 blue |
| Particle model
2 cards |
Key points of model
2. To print out as cards* on e.g.
green paper. |
Act
9 green |
| Ionic bonding
model A |
Key points of model
A. To print out as cards* on e.g.
red paper. |
Act
9 red |
| Ionic bonding
model B |
Key points of model
B. To print out as cards* on e.g.
yellow paper. |
Act
9 yellow |
| Phenomena cards |
A set of pages
with phenomena to be explained
with particle theory, to be printed
out as cards* |
Act
9 phenomena |
| Property cards |
A set of pages
with properties of NaCl to be
explained in terms of a model
of ionic bonding - to be printed
out as cards* |
Act
9 properties |
|
|
|
| |
| * Note: cards need to
be printed, e.g. 4 per page. |
| |
| Download
PDF of activity 9 brief |
| |
|